Answer:
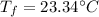
Step-by-step explanation:
Given:
initial temperature of the ice cube,
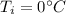
mass of the ice cube,
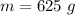
power of heating by the microwave oven,
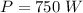
time duration of heating,
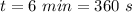
specific enthalpy of fusion of ice,
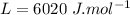
specific heat capacity of ice,
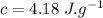
We find the energy supplied by the microwave:
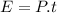
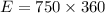
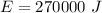
The energy required by the given mass of ice to melt:
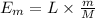
where:
M = molecular mass of the water
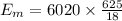
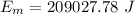
Remaining heat that is used in increasing the temperature of the melted ice i.e. water of 0°C:
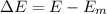
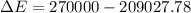
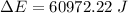
Now the rise in temperature of water:
From the heat equation,
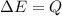
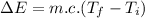
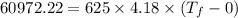
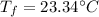 is the final temperature of the water after the given heat input.
is the final temperature of the water after the given heat input.