Step-by-step explanation:
As a neutral lithium atom contains 3 protons and its elemental charge is given as
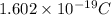 . Hence, we will calculate its number of moles as follows.
. Hence, we will calculate its number of moles as follows.
Moles =
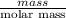
=
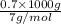
= 100 mol
According to mole concept, there are
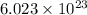 atoms present in 1 mole. So, in 100 mol we will calculate the number of atoms as follows.
atoms present in 1 mole. So, in 100 mol we will calculate the number of atoms as follows.
No. of atoms =
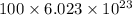
=
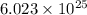 atoms
atoms
Since, it is given that charge on 1 atom is as follows.
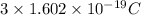
=
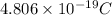
Therefore, charge present on
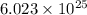 atoms will be calculated as follows.
atoms will be calculated as follows.
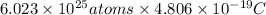
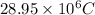
Thus, we can conclude that a positive charge of
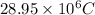 is in 0.7 kg of lithium.
is in 0.7 kg of lithium.