Answer:
322J
Step-by-step explanation:
The first law of thermodynamics states that "energy can neither be destroyed nor created but can be converted from one form to another with the interaction of heat, internal energy and work". Mathematically, this means that;
ΔU = Q + W ------------------------(i)
Where;
ΔU = total change in internal energy of the system
Q = heat exchanged between the system and its surrounding
W = work done by or on the system.
But,
Workdone is equal to the product of negative external pressure(p) on the system and change in volume (ΔV). i.e
W = -p x ΔV ---------------------------------(ii)
Substituting W = -p x ΔV into equation (i) gives;
ΔU = Q - pΔV ---------------------------(iii)
ΔV = final volume - initial volume
From the question,
final volume = 1.21L = 1.21
initial volume = 5.65L
=> ΔV = 1.21L - 5.65L
=> ΔV = -4.44L
Converting the result to
 gives;
gives;
-4.44L = -4.44 x
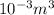 = -0.00444
= -0.00444

Also, from the question,
p = external pressure = 1.00atm;
Converting the pressure from atm to Pascal(Pa) gives;
1.00atm = 1.00 x 101.325 x
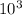 Pa = 101.325 x
Pa = 101.325 x
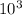 Pa
Pa
Also,
Q = heat exchanged = -128J (the negative sign shows that there is a loss of heat).
Now substituting the values of p, Q and ΔV into equation (iii) above gives;
=> ΔU = Q - pΔV
=> ΔU = -128 - (101.325 x
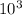 x -4.44 x
x -4.44 x
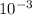 )
)
=> ΔU = -128 - (-449.883)
=> ΔU = -128 + 449.883
=> ΔU = 321.883J
=> ΔU = 322J (to 3 significant figures)
Therefore, the change in internal energy of the gas is 322J