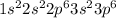Answer:
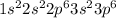
Step-by-step explanation:
Chlorine is the element of group 17 and third period. The atomic number of chlorine is 17 and the symbol of the element is Cl.
The electronic configuration of the element chlorine is:-
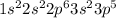
Chloride ion is formed when chlorine atom gain one more electron. So, the ground-state electron configuration for the chloride ion is:-