Answer: The boiling point of benzene at given external pressure is 162.45°C
Step-by-step explanation:
To calculate the boiling point of benzene, we use the Clausius-Clayperon equation, which is:
![\ln((P_2)/(P_1))=(\Delta H_(vap))/(R)[(1)/(T_1)-(1)/(T_2)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/wy1iixlq5kvfsdi78d17yq60bvb01nfu95.png)
where,
 = initial pressure which is the pressure at normal boiling point = 760 torr
= initial pressure which is the pressure at normal boiling point = 760 torr
 = final pressure which is external pressure = 5500 torr
= final pressure which is external pressure = 5500 torr
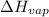 = Enthalpy of vaporization = 30.72 kJ/mol = 30720 J/mol (Conversion factor: 1 kJ = 1000 J)
= Enthalpy of vaporization = 30.72 kJ/mol = 30720 J/mol (Conversion factor: 1 kJ = 1000 J)
R = Gas constant = 8.314 J/mol K
 = initial temperature or normal boiling pont =
= initial temperature or normal boiling pont =
![80.1^oC=[80.1+273]K=353.1K](https://img.qammunity.org/2021/formulas/chemistry/high-school/e3mm2itg2sdopoi0bupsszlifyze16g8s9.png)
 = final temperature = ?
= final temperature = ?
Putting values in above equation, we get:
![\ln((5500)/(760))=(30720J/mol)/(8.314J/mol.K)[(1)/(353.1)-(1)/(T_2)]\\\\T_2=435.45K](https://img.qammunity.org/2021/formulas/chemistry/high-school/91to89sp8zfp11ibgc8uvvqcq334ppthl8.png)
Converting the temperature from kelvins to degree Celsius, by using the conversion factor:
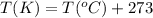
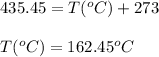
Hence, the boiling point of benzene at given external pressure is 162.45°C