Answer:
Molar mass of fructose’s empirical formula
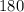 g/ mol
g/ mol
Step-by-step explanation:
The molecular formula of glucose is
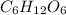 . The empirical formula of glucose is
. The empirical formula of glucose is
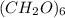
Like wise the molecular formula of fructose is
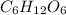 . The empirical formula of fructose is
. The empirical formula of fructose is
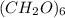
Empirical formula gives the proportionate mass of a chemical compound.
The molar mass of fructose is equal to the sum of individual masses of its constituting elements in a proportion as presented in empirical formula.
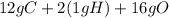
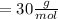
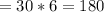 g.mol as there are 6 molecules of
g.mol as there are 6 molecules of
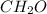 in fructose.
in fructose.