Answer: The given solution is unsaturated solution
Step-by-step explanation:
We are given:
Solubility of NaBr = 9.19 m
Unsaturated solution is defined as the solution in which more solute particles can be dissolved in the solvent.
Saturated solution is defined as the solution in which no more solute particles can be dissolved in the solvent.
A precipitate is defined as the insoluble salt which is formed when two solutions are mixed containing soluble substances. The insoluble salt settles down at the bottom of the reaction mixture.
There are three conditions:
- When
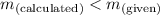 ; the solution is unsaturated
; the solution is unsaturated - When
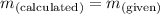 ; the solution is saturated
; the solution is saturated - When
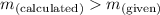 ; precipitate will form
; precipitate will form
To calculate the molality of solution, we use the equation:
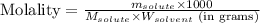
Where,
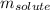 = Given mass of solute (sodium bromide) = 91.3 g
= Given mass of solute (sodium bromide) = 91.3 g
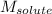 = Molar mass of solute (sodium bromide) = 103 g/mol
= Molar mass of solute (sodium bromide) = 103 g/mol
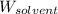 = Mass of solvent (water) = 115 g
= Mass of solvent (water) = 115 g
Putting values in above equation, we get:
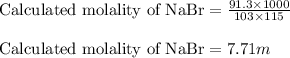
As, the calculated solubility is less than the given solubility. So, the solution will be unsaturated.
Hence, the given solution is unsaturated solution