Answer: The concentration of sulfate ions in the solution is 0.0522 M
Step-by-step explanation:
To calculate the the molarity of solution:, we use the equation:
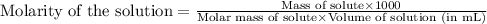
Given mass of alum = 2.17 g
Molar mass of alum = 478.39 g/mol
Volume of solution = 175 mL
Putting values in above equation, we get:
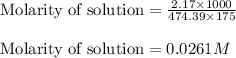
The chemical equation for the ionization of alum follows:
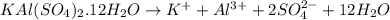
1 mole of alum produces 1 mole of potassium ions, 1 mole of aluminium ions, 2 moles of sulfate ions and 12 moles of water
So, concentration of sulfate ions =
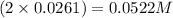
Hence, the concentration of sulfate ions in the solution is 0.0522 M