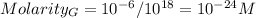Answer:

Step-by-step explanation:
For fully understanding of the question, I copy it adding some parts that are missing:
A stock solution of glucose is prepared by dissolving of 0.1802g of glucose in water using a 1-L volumetric flask (precision is (+/-) 0.1 mL ). A dilution (solution "A") is made by taking 1.00mL of the stock solution (using a pipet) and diluting to 1L with water (using a volumetric flask). This dilution procedure (dilute a 1-mL aliquot to 1L) is repeated six more times using the same glassware to prepare solution "B" through "G". what is the molarity of glucose solution G?
(end of the question)
Solution to the problem:
1. Calculate the molarity of the stock solution:
- Molar mass of glucose: 180.156 g/mol (from internet)
2. Calculate the molarity of the first dilution (solution A)
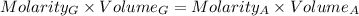
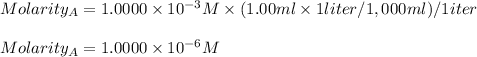
The solution was diluted by a factor of
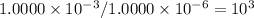
You can realize that every time that you dilute the solution, its molarity will dcrease by a factor of 10³.
Then, the dilutions is made 6 more times, you can divide the molarity of solution A by
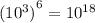 .
.
Hence, the molarity of solution G is: