Answer:
- Option B) All the ammonia is consumed
Step-by-step explanation:
1. Balanced chemical equation (given):
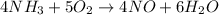
2. Theoretical mole ratios:
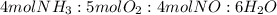
3. Limiting reactant:
When 1 mole of ammonia is combined with 2 moles of oxygen, the mole ratio is:
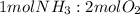
Hence, one of the reactants will be completely consumed (the limiting reactant) and, after completion, an excess of the other will remain unreacted.
You need to compare the the actual ratio with the theoretical ratio.
Hence, NH₃ is in less proportion with respect to oxygen than what is theoretically needed, and the former is the limiting reactant.
Therefore, the 1 mole (all) of ammonia will be consumed, while some oxygen will remain as excess. This is described by the option B) All the ammonia is consumed.
4. Analyze the other options:
The amount of oxygen that will react is:
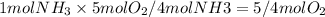
And the amount that will remain is:
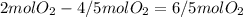
Neither option A) nor C) describe that situation.
The amount of water produced is:
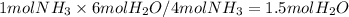 , which is not described by the option D).
, which is not described by the option D).
Hence, the correct answer is the option B) All the ammonia is consumed.