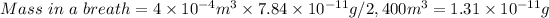Answer:
a) 7.9×10⁻¹¹ g (rounded to 2 significant figures)
b) 1.3×10⁻¹⁷ g (rounded to 2 significant figures)
Step-by-step explanation:
A) Mass of dust in the room
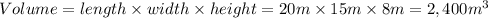
- Number of particles of dust in the room
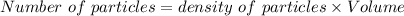
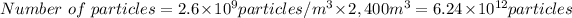
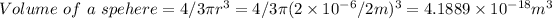
- Volume of 2.6×10⁹ particles
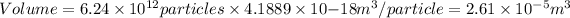
- Mass of 2.6×10⁹ particles
Density = 1.00 g/cm³ × sp gr = 1.00 g/cm³ × 3 = 3.00 g/cm³
Density = 3.00 g/cm³ × (1m/100cm)³ = 3.00×10⁻⁶ g/m³
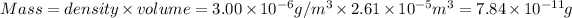
B) Mass of dust in each average breath of 400cm³
- Convert 400cm³ to m³: 4.00×10⁻⁴ m³
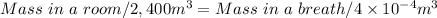
- Solve for Mass in a breath