The molarity of the solution is 1.69M.
Step-by-step explanation:
The reaction between the two reactants are:
CuNO₃+ NaI = CuI +NaNO₃.
So, 1 mole of CuNO₃ or copper nitrate produces 1 mole of CuI or Copper iodide.
Molecular weight of copper iodide =
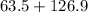 = 190.4.
= 190.4.
So, moles of copper iodide produced here is =
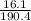 = 0.08.
= 0.08.
So, moles of copper nitrate that was present in the solution = 0.08.
Amount of solution used = 50ml.
So, in 50ml or solution, moles of copper nitrate present = 0.08 moles.
So, in 1 liter of solution, moles of copper nitrate present = 1.69 moles.
So, the molarity of the solution is 1.69M.