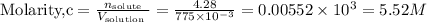The molarity of a salt solution made by dissolving 250.0 grams of NaCl in 775 mL of solution is 5.52 M .
Step-by-step explanation:
The molarity of a solution tells one how many moles of solute one can get per liter of solution.One should remember to must convert this volume to liters by using the conversion factor:
1 L = 10³ml
Sodium chloride (NaCl) has a molar mass of 58.44 g per mol , which means that the sample will contain:
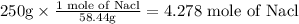
This means that the molarity of the solution will be: