Answer: Activation energy
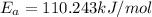
Step-by-step explanation:
Arrhenius expression activation energy is given by
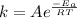
where
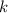 is the rate constant,
is the rate constant,
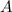 is the pre-exponential factor
is the pre-exponential factor
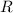 =The gas constant is 8.314 J / mol·K and
=The gas constant is 8.314 J / mol·K and
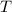 is temperature
is temperature
By substituting the values in the above equation we get two equations

by solving 1 and 2, we get
![1.5* 10^(-3)=\frac{e^{(-E_(a))/(8.314* 600)}}{e^{(-E_(a))/(8.314* 850)}}\\1.5* 10^(-3)\tiimes {e^{(-E_(a))/(8.314* 850)}={e^{(-E_(a))/(8.314* 600)}\\ln(1.5* 10^(-3))\tiimes+ln( {e^{(-E_(a))/(8.314* 850)})=ln({e^{(-E_(a))/(8.314* 600)})\\-6.50+ -E_(a)/(8.314* 850)={(-E_(a))/(8.314* 600)\\E_(a)=110.243 kJ/mol]()