Answer:
500-g gold cup > 500-g cast iron cup > 750-g cast iron cup
Step-by-step explanation:
From the heat energy equation we know that:
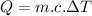
where:
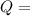 heat transfer from or to the body
heat transfer from or to the body
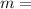 mass of the body concerned
mass of the body concerned
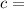 specific heat of the material of the concerned body
specific heat of the material of the concerned body
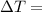 temperature difference between the bodies in contact
temperature difference between the bodies in contact
Since the gain in temperature of the cup is proportional to the amount of heat which the water holds and is inversely proportional to the mass of the cup and the specific heat capacity of the material.
We have the specific heat of gold less than the specific heat of the cast iron therefore for the same mass of gold and cast iron we require lesser heat in case of gold to increase its temperature by 1 kelvin.