At 25°C,

= 2.9 x 10⁻³ for the following reaction.
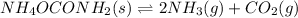
In an experiment carried out at 25°C, a certain amount of NH₄OCONH₂ is placed in an evacuated rigid container and allowed to come to equilibrium.
Calculate the total pressure in the container at equilibrium.