Final answer:
The net charge on the substance is

Step-by-step explanation:
The net charge on a substance is the difference between the total number of protons and electrons.
In this case, the substance consists of
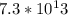 protons and
protons and
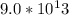 electrons.
electrons.
Since protons have a positive charge and electrons have a negative charge, we can calculate the net charge by subtracting the number of electrons from the number of protons.
The net charge =

After calculating, the net charge is
