Answer:
The answer to your question is P = 1.357 atm
Step-by-step explanation:
Data
Volume = 22.4 L
1 mol
temperature = 100°C
a = 0.211 L² atm
b = 0.0171 L/mol
R = 0.082 atmL/mol°K
Convert temperature to °K
Temperature = 100 + 273
= 373°K
Formula
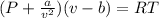
Substitution
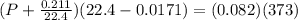
Simplify
(P + 0.0094)(22.3829) = 30.586
Solve for P
P + 0.0094 =
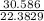
P + 0.0094 = 1.366
P = 1.336 - 0.0094
P = 1.357 atm