Answer:
The body temperature would rise by 47.85 °C
The amount of water the body evaporates is 4.15 kg.
This makes sense because firstly the value obtained is positive then secondly it is a normal occurrence in the real world that in a place where the temperature is high the body usually produce sweat in order to balance its internal temperature
Step-by-step explanation:
Considering the relationship (between the heat released and the mass of the object) as shown below
q = msΔT
where q is the heat released per day =
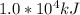
m is the mass of the body = 50 kg
ΔT is the temperature rise = ?
s is the specific heat of water =
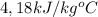
substituting values we have
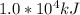 =
=
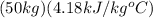 ΔT
ΔT
ΔT =
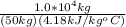
= 47.85°C
To maintain the normal body temperature (98.6F = 37°C) the amount of heat released by metabolism activity must be utilized for evaporation of some amount of water
Hence
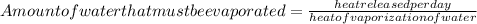
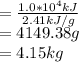
Note (1 kg = 1000 g)
This makes sense because firstly the value obtained is positive then secondly it is a normal occurrence in the real world that in a place where the temperature is high the body usually produce sweat in order to balance its internal temperature