Step-by-step explanation:
It is true that inside every nucleus of an atom there will be protons and neutrons. And, electrons will revolve around the nucleus of the atom.
And, these electrons are actually located as pairs inside the orbital. As they are present in pairs so they have
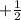 and
and
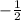 spin.
spin.
For example, orbitals are located in sub-shells like 1s, 2s, 2p etc.
Thus, we can conclude that atoms are composed of a nucleus made from protons and neutrons surrounded by clouds of electrons. Those electrons are organized into layers. Within these layer, electrons are further organized as pairs in orbitals.