Complete Question
The complete question is shown on the first uploaded image
Answer:
The structure of Biphenyl and Methyl Orange is shown on the second uploaded image
Looking at the structures of both compound we can see that methyl orange is a polar compound due to the presence of -SO₃Na functional group. This -SO₃Na group is ionic in nature and imparts polarity to the methyl orange , while for biphenyl is a non-polar compound due to the absence of the polar functional groups in its structure
The technique silica gel chromatography is an excellent tool for the separation of molecules based on polarity differences.Silica gel(stationary state)is polar in nature.This means that non polar compounds tend to filter before more polar ones.looking at the case at hand biphenyl will filter first due to its non-polar nature and therefore would have high
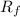 value on TCL(Thin-layer chromatography) plate
value on TCL(Thin-layer chromatography) plate
NOTE :
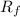 means (The distance traveled by sample )/(The distance traveled by solvent(in its mobile phase)).
means (The distance traveled by sample )/(The distance traveled by solvent(in its mobile phase)).
Step-by-step explanation:
In order to get a better understanding let explain how TCL(Thin-layer chromatography) works
The TCL plate (stationary phase) is made of glass or plastic that is been coated by adsorbent material in this case of this experiment a silica gel
Step one of the procedure is to apply the non- volatile mixture on the TCL plate then by capillary action the solvent(mobile phase which is mostly heptane a non-polar compound) is drawn up from the bottom of the plate to the top, because different molecules ascend the plate at different rates ,separation is achieved.