Step-by-step explanation:
Formula for change in the potential energy of the ammonia molecule is as follows.
U =
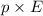
Here, dipole moment is p and electric field is E.
Putting
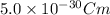 for p and
for p and
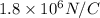 for E in the above equation is as follows.
for E in the above equation is as follows.
U =
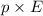
=
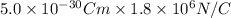
=
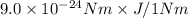
=
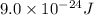
Therefore, we can conclude that the change in the potential energy of the ammonia molecule is
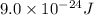 .
.