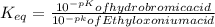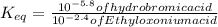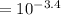Answer:
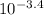
Step-by-step explanation:
Let us first take a look at the image below;
In the acid - base reaction; we can see the transfer of electrons that takes place;
We can also see that the reaction goes in the direction which converts the stronger acid and the stronger base to the weaker acid and the weaker base.
The stronger acid is shown with the one with more negative
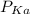 Value.
Value.
∴ The equilibrium constant for the acid-base reaction is expressed as:
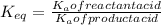

From
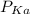 Value (shown in the image below), it is clear and vivid that hydrobromic acid is a stronger acid than the ethyloxonium ion, therefore the equilibrium lies to the right.
Value (shown in the image below), it is clear and vivid that hydrobromic acid is a stronger acid than the ethyloxonium ion, therefore the equilibrium lies to the right.
From the chemical equation (shown in the attached image); the equilibrium constant for the acid-base reaction can be expressed as: