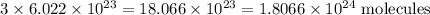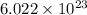 molecule(s) of nitrogen and
molecule(s) of nitrogen and
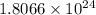 molecules of hydrogen react with each other to form
molecules of hydrogen react with each other to form
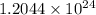 molecules of ammonia.
molecules of ammonia.
Step-by-step explanation:
Given:
The chemical equation for producing ammonia from nitrogen and hydrogen
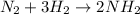
1 mole of nitrogen gets reacted with 3 moles of hydrogen in order to form 2 moles of ammonia.
One mole =
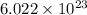 (Applicable to the substances like ions, molecules, or atoms). This specified number is called as Avogadro's constant or number. This idea helps us to convert between the number of particle and mass.
(Applicable to the substances like ions, molecules, or atoms). This specified number is called as Avogadro's constant or number. This idea helps us to convert between the number of particle and mass.
Therefore, when dealing this with the given chemical equation,
1 mole of nitrogen =
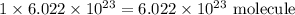
2 moles of ammonia =
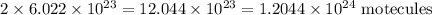
3 moles of hydrogen =