Answer:
36 g ammonia (NH₃) is required to react with 34 g methanol (CH₃OH)
Step-by-step explanation:
The chemical equation for the described chemical reaction:
CH₃OH + 2NH₃ → product
In this chemical reaction, 2 moles of ammonia (NH₃) reacts with 1 mole of methanol (CH₃OH) to give the product.
Also, the molar mass of methanol = 32 g/mol,
and, the molar mass of ammonia = 17 g/mol
As we know, number of moles = given mass ÷ molar mass
or, given mass = number of moles × molar mass
∴ In the given reaction, the mass of ammonia reacting = 2 mole × 17 g/mol = 34 g
And, the mass of methanol reacting = 1 mole × 32 g/mol = 32 g
Now, to find the amount of ammonia that will react with 34 g methanol:
As, in the given reaction,
34 g ammonia reacts with 32 g methanol
So, the amount of ammonia that reacts with 34 g methanol =
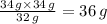
Therefore, 36 g ammonia (NH₃) is required to react with 34 g methanol (CH₃OH).