Answer: The concentration of salt (potassium phosphate), phosphoric acid and KOH in the solution is 0.0342 M, 0.0533 M and 0 M respectively.
Step-by-step explanation:
To calculate the number of moles for given molarity, we use the equation:
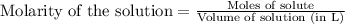 .....(1)
.....(1)
For KOH:
Initial molarity of KOH solution = 0.205 M
Volume of solution = 25.0 mL = 0.025 L (Conversion factor: 1 L = 1000 mL)
Putting values in equation 1, we get:
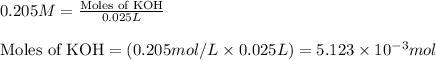
For phosphoric acid:
Initial molarity of phosphoric acid solution = 0.175 M
Volume of solution = 25.0 mL = 0.025 L
Putting values in equation 1, we get:
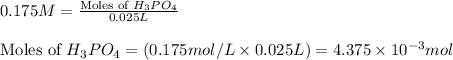
The chemical equation for the reaction of KOH and phosphoric acid follows:
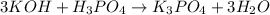
By Stoichiometry of the reaction:
3 moles of KOH reacts with 1 mole of phosphoric acid
So,
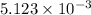 moles of KOH will react with =
moles of KOH will react with =
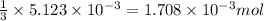 of phosphoric acid
of phosphoric acid
As, given amount of phosphoric acid is more than the required amount. So, it is considered as an excess reagent.
Thus, KOH is considered as a limiting reagent because it limits the formation of product.
Excess moles of phosphoric acid =
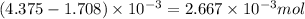
By Stoichiometry of the reaction:
3 moles of KOH produces 1 mole of potassium phosphate
So,
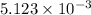 moles of KOH will produce =
moles of KOH will produce =
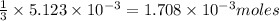 of potassium phosphate
of potassium phosphate
Moles of potassium phosphate =
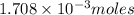
Volume of solution = [25.0 + 25.0] = 50.0 mL = 0.050 L
Putting values in equation 1, we get:
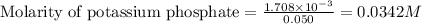
Moles of excess phosphoric acid =
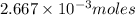
Volume of solution = [25.0 + 25.0] = 50.0 mL = 0.050 L
Putting values in equation 1, we get:
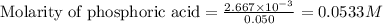
Moles of KOH remained = 0 moles
Volume of solution = [25.0 + 25.0] = 50.0 mL = 0.050 L
Putting values in equation 1, we get:
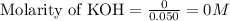
Hence, the concentration of salt (potassium phosphate), phosphoric acid and KOH in the solution is 0.0342 M, 0.0533 M and 0 M respectively.