Answer:
P_2 = 62.69 psi
Step-by-step explanation:
given,
P₁ = 70 psia T₁ = 55° F = (55 + 459.67) R
P₂ = ? T₂ = 115° F = (115 + 459.67) R
we know,
p = ρ RT
ρ is the density which is constant
R is also constant
now,
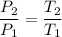
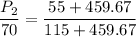
P_2 = 62.69 psi
Hence, the increase in Pressure is equal to P_2 = 62.69 psi