Answer:
temperature does the average speed of an oxygen molecule equal that of an airplane moving at 590 mph = 89.24 K
Step-by-step explanation:
Average speed of oxygen molecule is given by
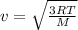
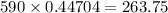 m/s
m/s
R= 8.314 J/mol K = universal gas constant
M= molecular weight of oxygen = 32 g/mol =0.032 Kg/mol
now plugging these values to find T we get
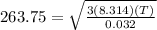
solving the above equation we get
T= 89.24 K