Answer:
4.98 × 10⁻³ mol
Step-by-step explanation:
Given data for Cl₂
- Volume (V): 115 mL = 0.115 L
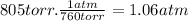
- Temperature (T): 25°C + 273.15 = 298 K
First, we will calculate the moles of Cl₂ using the ideal gas equation.
P × V = n × R × T
n = P × V / R × T
n = 1.06 atm × 0.115 L / (0.0821 atm.L/mol.K) × 298 K
n = 4.98 × 10⁻³ mol
Let's consider the balanced equation.
MnO₂(s) + 4 HCl(aq) ⟶ MnCl₂(aq) + 2 H₂O(l) + Cl₂(g)
The molar ratio of MnO₂ to Cl₂ is 1:1. The required moles of MnO₂ are 4.98 × 10⁻³ moles.