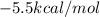Answer: b)
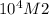 and
and
Step-by-step explanation:
Equilibrium concentration of
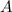 =
=
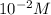
Equilibrium concentration of
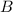 =
=
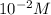
Equilibrium concentration of
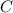 =
=
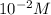
Equilibrium concentration of
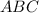 =
=
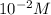
The given balanced equilibrium reaction is,
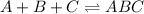
The expression for equilibrium constant for this reaction will be,
![K_c=([ABC])/([A][B][C])](https://img.qammunity.org/2021/formulas/biology/college/78stx5krp7yt92d4atmdis1s0s4g5t0i51.png)
Now put all the given values in this expression, we get :
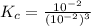
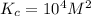
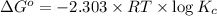
where,
R = universal gas constant = 2 cal/K/mole
T = temperature = 300 K
 = equilibrium constant =
= equilibrium constant =
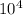
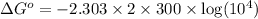
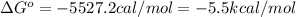
Thus the equilibrium constant and the standard free energy of this association reaction at T=300 K are
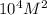 and
and