Answer:
0.0257259766982 m
Step-by-step explanation:
 = Atmospheric pressure = 101325 Pa
= Atmospheric pressure = 101325 Pa
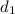 = Initial diameter = 1.5 cm
= Initial diameter = 1.5 cm
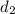 = Final diameter
= Final diameter
 = Density of water = 1000 kg/m³
= Density of water = 1000 kg/m³
h = Depth = 40 m
The pressure is
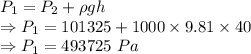
From ideal gas law we have
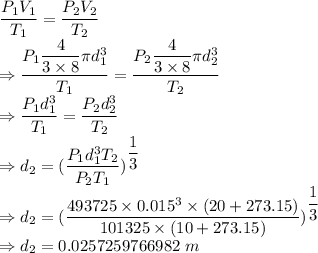
The diameter of the bubble is 0.0257259766982 m