Answer:
The answer to your question is T2 = 20.93°K
Step-by-step explanation:
Data
Volume 1 = V1 = 2.8 m³
Temperature 1 = T1 = 20°C
Volume 2 = V2 = 0.2 m³
Temperature 2 = T2 = ?
Process
To solve this problem use Charles' law
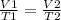
Solve for T2
T2 =
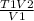
1.- Convert temperature to °K
T1 = 20 + 273 = 293°K
2.- Substitute values
T2 =
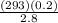
3.- Simplify
T2 =
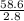
4.- Result
T2 = 20.92°K