Answer : The concentration of a solution with an absorbance of 0.420 is, 0.162 M
Explanation :
Using Beer-Lambert's law :

As per question, at constant path-length there is a direct relation between absorbance and concentration.

where,
A = absorbance of solution
C = concentration of solution
l = path length
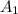 = initial absorbance = 0.350
= initial absorbance = 0.350
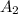 = final absorbance = 0.420
= final absorbance = 0.420
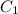 = initial concentration = 0.135 M
= initial concentration = 0.135 M
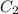 = final concentration = ?
= final concentration = ?
Now put all the given value in the above relation, we get:
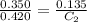
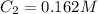
Thus, the concentration of a solution with an absorbance of 0.420 is, 0.162 M