Answer:
11.02 % of an isotope will be left after 45 seconds.
Step-by-step explanation:
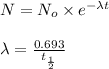
where,
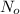 = initial mass of isotope
= initial mass of isotope
N = mass of the parent isotope left after the time, (t)
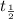 = half life of the isotope
= half life of the isotope
 = rate constant
= rate constant
We have :
Mass of Beryllium-11 radioactive isotope=

Mass of Beryllium-11 radioactive isotope after 45 seconds =
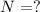
t = 45 s
 = rate constant =
= rate constant =
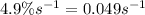
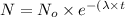
Now put all the given values in this formula, we get
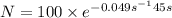
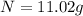
Percentage of isotope left :
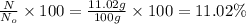
11.02 % of an isotope will be left after 45 seconds.