Answer:
The answer to the question is
The mass percentage of NaCl(s) in the mixture is 49.7%
Step-by-step explanation:
The given variables are
mass of sample of mixture = 0.3146 g
Volume of AgNO₃ required to react comletely with the chloride ions = 45.70 mL
Concentration of the AgNO₃ added = 0.08765 M
The equations for the reactions oare
NaCl(aq) + AgNO₃ (aq) = AgCl(s) + NaNO₃(aq)
AgNO₃ (aq) + KBr (aq) → AgBr (s) + KNO₃
The equation for the reaction shows one mole of NaCl reacts with one mole of AgNO₃ to form one mole of AgCl
Thus 45.70 mL of 0.08765 M solution of AgNO₃ contains
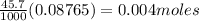
Therefore the sum of the number of moles of Br⁻ and Cl⁻
precipitated out of the solution = 0.004 moles
Thus if the mass of NaCl in the sample = z then the mass of KBr = y
However the mass of the sample is given as 0.3146 g which means the molarity of the solution is 0.004 moles
given by
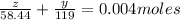 and z + y = 0.3146
and z + y = 0.3146
Therefore z = 0.3146 - y which gives
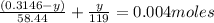
-8.7×10⁻³y +0.54×10⁻³ = 0.004
or 8.7×10⁻³y = 1.37769× 10⁻³
y = 0.158 g and z = 0.156 Thus the mass of NaCl = 0.156 g and the mass percentage = 0.156/0.3146×100 = 49.7% NaCl
The mass percentage of NaCl(s) in the mixture is 49.7%