Answer: 10 ml of 200 mM
 is required and 30 ml of water is required.
is required and 30 ml of water is required.
Step-by-step explanation:
According to the dilution law,
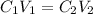
where,
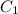 = concentration of stock solution = 200mM
= concentration of stock solution = 200mM
 = volume of stock solution = ?
= volume of stock solution = ?
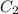 = concentration of resulting solution= 50mM
= concentration of resulting solution= 50mM
 = volume of another acid solution= 40 ml
= volume of another acid solution= 40 ml
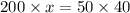
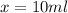
Thus 10 ml of 200 mM
 is required and (40-10) ml = 30 ml of water is to be added to make 40 ml of 50mM
is required and (40-10) ml = 30 ml of water is to be added to make 40 ml of 50mM
 .
.