Answer:
The net work produced = -36737.52 J
The efficiency of the cycle = 72.2%
Step-by-step explanation:
Given that :
The temperature of the hot reservoir
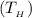 = 800 °C = (800+273)K
= 800 °C = (800+273)K
The temperature of the cold reservoir
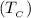 = 25 °C (25+273)K
= 25 °C (25+273)K
Pressure
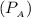 = 0.2 bar
= 0.2 bar
Pressure
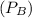 = 60 bar
= 60 bar
Rate constant (R) = 8.314
Determination of the efficiency of the cycle (η) is given by the formula:
(η) =
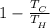
= 1 -
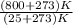
= 0.722
= 72.2 %
∴ The efficiency of the cycle = 72.2 %
However, the heat given along the initial hot isothermal path
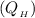 is equal to the work done which is given by the equation;
is equal to the work done which is given by the equation;
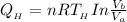
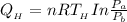
substituting our data from the given parameters above; we have:
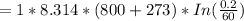
= -50882.99 J
To determine the net work produced; we have:
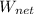 = η
= η
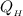
= 0.722 × (-50882.99 J)
= -36737.52 J
∴ The net work produced = -36737.52 J