Answer: 0.057 kg
Step-by-step explanation:
We can find the mass of the oil if we know its density and the volume it occupies. Since the density
 is a relation between the mass
is a relation between the mass
 and the volume
and the volume
 of a substance:
of a substance:
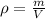 (1)
(1)
Now, the density of oil is generally between
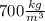 and
and
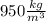 . However, in this case we will take
. However, in this case we will take
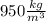 as its density.
as its density.
In adition, we are given as data the volume thw oil occupies:
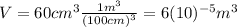
Writing these values in (1):
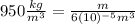 (2)
(2)
Isolating
 :
:
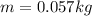 This is the mass of the oil
This is the mass of the oil