The final pressure will be 405.3 kilopascals.
Step-by-step explanation:
According to the Boyle's Law, the pressure of the gas is inversely proportional to the volume of the gas as the temperature of the gas remains constant.
So,PV = Constant.
Here in this question, the volume of the container is halved.
Let the Pressure of the gas initially be P1 and final pressure be P2. Volume of gas initially be V
So, according to Boyle's Law,
P₁V₁ = P₂V₂PP.
So,
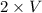 = P₂
= P₂
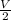 .
.
So, P2 = 4 atm.
1atm = 101.325 kilo pascals.
So, 4 atm =
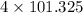 kilopascals. = 405.3 kilopascals.
kilopascals. = 405.3 kilopascals.