Answer:
T₂ = 111.57 °C
Step-by-step explanation:
Given that
Initial pressure P₁ = 9.8 atm
T₁ = 32°C = 273 + 32 =305 K
The final pressure P₂ = 11.2 atm
Lets take the final temperature = T₂
We know that ,the ideal gas equation
If the volume of the gas is constant ,then we can say that
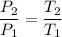
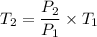
Now by putting the values in the above equation ,we get
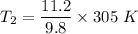
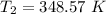
T₂ = 384.57 - 273 °C
T₂ = 111.57 °C