Answer:
4.58 L.
Step-by-step explanation:
Given that
V₁ = 5 L
T₁ = 25°C = 273 + 25 = 298 K
T₂ = 0°C = 273 K
The final volume = V₂
We know that ,the ideal gas equation
If the pressure of the gas is constant ,then we can say that
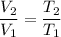
Now by putting the values in the above equation we get
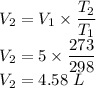
The final volume of the balloon will be 4.58 L.