Answer : The mass of solute needed are, 40 grams.
Explanation :
As we are given that 40 % (w/v) solution of sodium bicarbonate (solute) that means 40 grams of sodium bicarbonate present in 100 mL of solution.
Now we have to calculate the mass of solute needed.
As, 100 mL of solution needs mass of solute = 10 g
So, 400 mL of solution needs mass of solute =
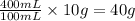
Thus, the mass of solute needed are, 40 grams.