Answer:
a. Shift towards product side
b. Shift towards reactant side
c. Shift towards product side
Step-by-step explanation:
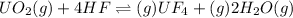
Any change in the equilibrium is studied on the basis of Le-Chatelier's principle.
This principle states that if there is any change in the variables of the reaction, the equilibrium will shift in the direction to minimize the effect.
- Adding reactant at the equilibrium, will shift the equilibrium reaction in forward direction that is in right direction.
- Adding product at the equilibrium, will shift the equilibrium reaction in backward direction that is in left direction.
a.Uranium dioxide is added.
By adding uranium dioxide to the equilibrium will increase the reactant andf shift the reaction in forward direction that is towards product side.
b. Hydrogen fluoride reacts with the walls of the reaction vessel.
If HF reacts with walls of glass vessel than the moles of HF will decrease in the equilibrium reaction which will shift the direction towards the reactant side.
c. Water vapor is removed.
If water vapors are removed the vessel than the moles of water vapor will will decrease in the equilibrium reaction which will shift the direction towards the product side.