Answer:
λ = 397 nm
Step-by-step explanation:
given,
Rydberg wavelength equation for Balmer series
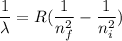
R is the Rydberg constant, R = 1.097 x 10⁷ m⁻¹
n_i = initial energy level
n_f = final energy level
where as for Balmer series n_f = 2
n_i = 7
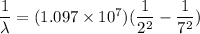
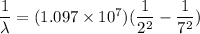
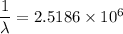
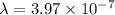
Hence, the wavelength is equal to λ = 397 nm