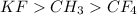Step-by-step explanation:
Ionic compounds have high boiling point due to the presence of strong intermolecular forces present within the molecules due to the partial opposite charges on its atoms.
For example, KF is an ionic compound so it has high boiling point.
Whereas covalent compounds have weak forces because of sharing of electrons taking place between the molecules of a substance.
Whereas
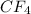 is a covalent compound as both the atoms are non-metals. Hence, it has low boiling point.
is a covalent compound as both the atoms are non-metals. Hence, it has low boiling point.
In
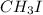 , there is dipole moment. Hence, it will have a boiling point greater than
, there is dipole moment. Hence, it will have a boiling point greater than
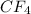 .
.
Therefore, we can conclude that order of decreasing boiling point of given compounds is as follows.