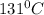Answer:
404K
Step-by-step explanation:
Data given, Kinetic Energy.K.E=8.37*10^-21J
Note: as the temperature of a is increase, the rate of random movement will increase, hence leading to more collision per unit time. Hence we can say that the relationship between the kinetic energy and the temperature is a direct variation.
This relationship can be expressed as
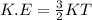
where K is a constant of value 1.38*10^-23
Hence if we substitute the values, we arrive at
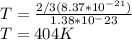
converting to degree we have