Answer : The pH of the solution is, 0.932
Explanation :
First we have to calculate the moles of HCl and NaOH.
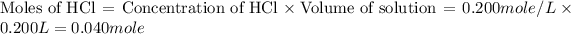
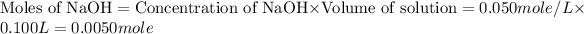
The balanced chemical reaction will be,

From the balanced reaction we conclude that,
As, 1 mole of NaOH neutralizes by 1 mole of HCl
So, 0.0050 mole of NaOH neutralizes by 0.0050 mole of HCl
Thus, the number of neutralized moles = 0.0050 mole
Remaining moles of HCl = 0.040 - 0.0050 = 0.035 moles
Total volume of solution = 200.0 mL + 100.0 mL = 300.0 mL = 0.300 L
Now we have to calculate the concentration of HCl(acid).
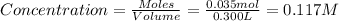
As we know that, 1 mole of HCl dissociates to give 1 mole of hydrogen ion and 1 mole of chloride ion.
So, concentration of
 = 0.117 M
= 0.117 M
Now we have to calculate the pH of solution.
![pH=-\log [H^+]](https://img.qammunity.org/2021/formulas/chemistry/high-school/rjo2yhb5oj9ry1fr4db1ujrazm6fh3vhke.png)
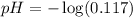
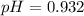
Thus, the pH of the solution is, 0.932