Answer:
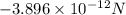 is an electric force on the potassium ion due to the chloride ion.
is an electric force on the potassium ion due to the chloride ion.
Step-by-step explanation:
Charge on potassium ion =
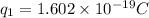
Charge on chlorine ion =
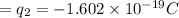
Separation between these two charges = r =
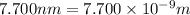
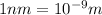
Electric force on the potassium ion due to the chloride ion = F
Coulomb's law is given as ;
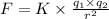
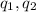 = Charges on both charges
= Charges on both charges
r = distance between the charges
K = Coulomb constant =
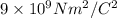
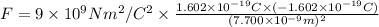
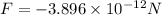
(negative sign indicates that attractive force is exerting between two ions)
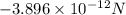 is an electric force on the potassium ion due to the chloride ion.
is an electric force on the potassium ion due to the chloride ion.