Answer:
Solution with 35.0 g of
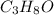 in 250.0 g of ethanol will have lowest freezing point
in 250.0 g of ethanol will have lowest freezing point
Step-by-step explanation:
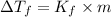
where,
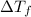 =depression in freezing point =
=depression in freezing point =
 = freezing point constant
= freezing point constant
m = molality
As we can see that higher the molality of the solution more will depression in freezing point of the solution and hence lower will the freezing point of solution.
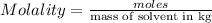
A. 35.0 g of
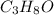 in 250.0 g of ethanol.
in 250.0 g of ethanol.
Moles of
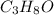 =
=
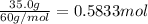
Mass of solvent i.e. ethanol = 250.0 g = 0.25 kg (1 g = 0.001 kg)
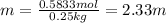
B. 35.0 g of
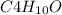 in 250.0 g of ethanol
in 250.0 g of ethanol
Moles of
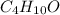
/(74 g/mol)=0.4730 mol](https://img.qammunity.org/2021/formulas/chemistry/college/br3up2yy2ru0n8q0w8t0i75ujcayh6krnx.png)
Mass of solvent i.e. ethanol = 250.0 g = 0.25 kg (1 g = 0.001 kg)
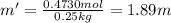
C. 35.0 g of
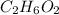 in 250.0 g of ethanol
in 250.0 g of ethanol
Moles of
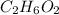 =
=
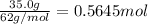
Mass of solvent i.e. ethanol = 250.0 g = 0.25 kg (1 g = 0.001 kg)

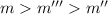
Solution with 35.0 g of
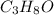 in 250.0 g of ethanol will have lowest freezing point
in 250.0 g of ethanol will have lowest freezing point