Answer:
 is the charge on each sphere.
is the charge on each sphere.
Step-by-step explanation:
Coulomb's law is given as ;
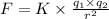
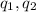 = Charges on both charges
= Charges on both charges
r = distance between the charges
K = Coulomb constant =
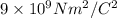
We have ;
Charge of ion =
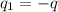
Charge of electron =
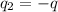
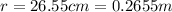
Force between the charges at r distance will be : F
F = 0.03500 N
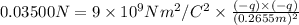
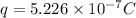
 is the charge on each sphere.
is the charge on each sphere.